Course Description
What if you are born with a condition, which very few people know about and for which there are no cures? Or what if there is a cure, but it is very expensive and you have to take it throughout your life? How can you encourage pharmaceutical industry invest in such cures and have policy makers consider such conditions when they draft new regulations? Rare diseases are not rare. There are 7000 diseases, but in aggregate, these diseases affect 30 million (i.e. 1 in 10) Americans of all ages and additional millions of people globally. Most of these conditions are serious and life-altering and children account for more than 50% of those affected. However, only 5% of all rare diseases have FDA-approved treatments. Thus, there is a large unmet need in this area and one way to address this is to raise awareness about these conditions.
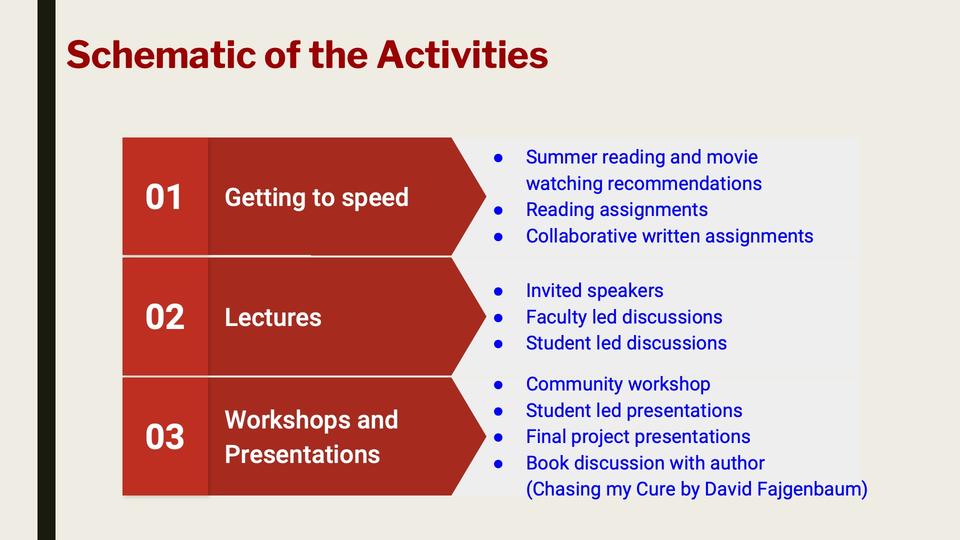
This course will comprise of weekly seminars and related readings on topics related to the understanding of rare diseases and the economics, regulatory and public policy aspects of development of drugs (orphan products) to treat these conditions in the US and across the globe. In this highly interactive course, students will learn from and network with researchers, healthcare professionals and business leaders and gain sufficient background to appreciate the scope of this multidisciplinary field. Students will work in teams with a patient advocacy organization to learn firsthand the challenges related to the diagnosis and treatment of a specific rare disease, barriers to research and development and deliver possible solutions to a specific challenge that they have identified.
15 Weeks of Learning
19 guest speakers + 4 community organizations
-
11 patients /Rare disease advocates
-
6 faculty
-
4 local companies
-
1 pharma
-
1 policymaker
|
Module |
Topics |
|
1: Introduction |
Topic Introduction (Definitions for rare disease/orphan drugs) |
|
2. Rare Diseases |
Diagnostic Odyssey |
|
3: Orphan Drugs |
Regulatory Aspects: |
|
4: Clinical Trials in Rare Diseases |
Current Therapies: Developing Novel therapies vs. Drug Repurposing |
|
5: Community Engagement |
How to Make a Difference in the Community |
Community Workshop
| Patient Advocacy Groups | Student Groups | Final Projects |
| Sickle Cell Foundation of Minnesota | Rare Disease Responders | Sickle Cell warriors video |
| Krabbe Connect | Zebras | Infographics: |
| Organic Acidemia Foundation | MN Crew | Infographic on Organic Acidemia Clinical Trials |
| Kasners Kick Duchenne | U Girls | Flyer promoting a book by Kasners Kick Duchenne |
| United Mitochondrial Disease Foundnation | Blue Genes | Mito Travel |
| Organic Acidemia Association | The Babinski's | Social Media Campaign |
| Yaya Foundation | Chasing Zebras | Yaya's Story |
| Sickle Cell Disease (SCD) Foundation of MN | Disease Destroyers | Sickle Cell Advocacy & Blood Donation Awareness Video | PDF |
| MN Rare Disease Advisory Council | Finding the Zebras | Rare Disease Advisory Council |
| Sickle Cell Foundation of MN | Butterscotch Blondies | Sickle Cell Disease Infographic and video |
| Organic Acidemia Association | Flower Power | GA type 1 and MMA presentations and videos |
| United Mitochondrial Disease Foundation | Rallying the Rare | Rallying The Rare videos |